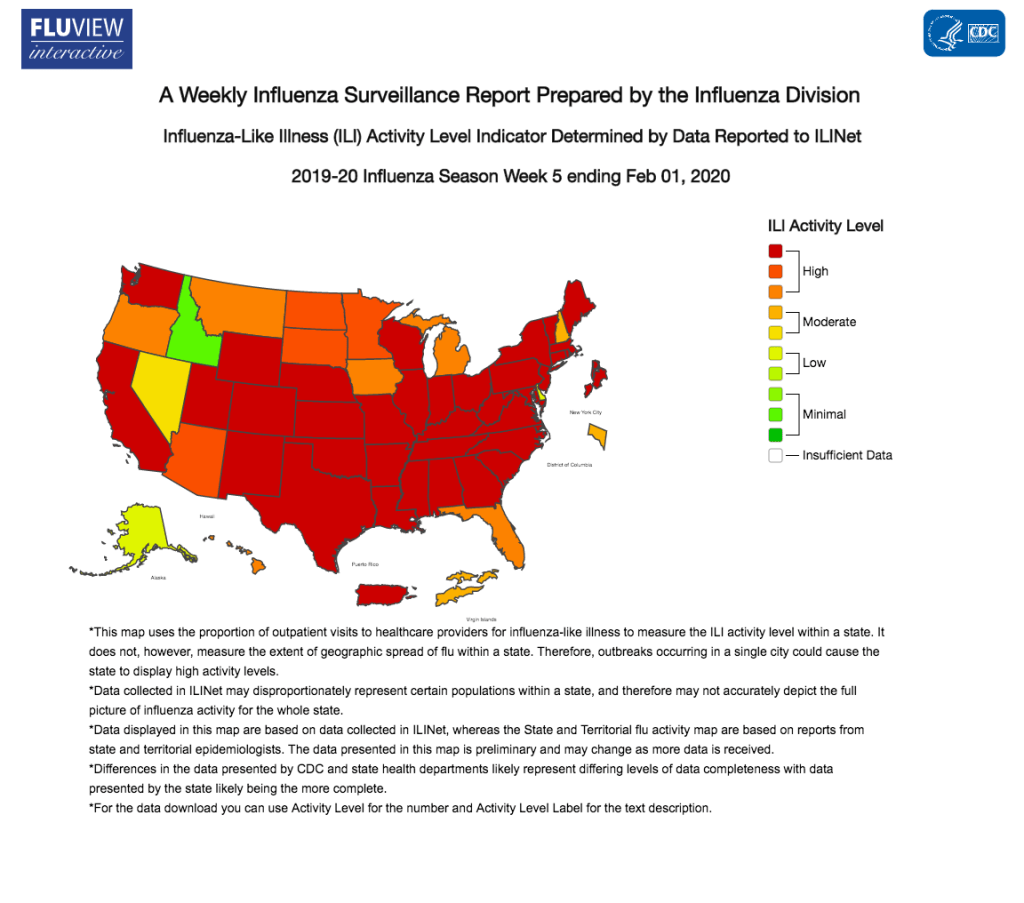
Here we are, back to school after summer and then after Christmas break and rather than enjoying ourselves and our classes we must worry about…the flu. The flu spreads every single year and causes death and yet people still do not get their vaccine and this in turn jeopardizes those who are unable to get it. The Centers for Disease Control and Prevention (CDC) provides weekly updates of the 2019-2020 flu season and we are currently in week 5 of data collection. The CDC estimates that at least 22 million people have been infected from the flu in the 2019-2020 season and 12,000 people have died from it. There are 4 types of the influenza virus and they are called A, B, C, or D. A and B are the viruses found in humans while C and D only cause mild flu symptoms or are observed in other animals such as cattle. However, there are hundreds of subtypes, clades, and sub-clades of the flu and this virus is able to change each year with antigenic shift. This causes the flu vaccine to be helpful but not necessarily prevent the flu because it does not exactly match the exact flu each year.
A policy statement from the American Academy of Pediatrics released recommendations for the 2019-2020 flu season in October of 2019. The 2 types of flu vaccine include the inactivated influenza vaccine (IIV) and the live attenuated influenza vaccine (LAIV) and everyone over the age of 6 months is recommended to receive a flu vaccine. This statement provides information on the vaccines that are most safe for each age group and targets hesitations to receiving the vaccine such and with immunocompromised individuals or those with egg allergies. Is recommended that those who are in close contact with immunocompromised individuals to either receive the IIV vaccine or wait at least 7 days after receiving the LAIV before coming into contact with those individuals. It also states that those will an egg allergy can safely receive either vaccine so this should be not a barrier.
The CDC also reports of the effectiveness of the flu vaccines each year. For this year, the overall effectiveness is 38%. The vaccines are predicted to be 62% effective against the A[H1N1]pdm09 virus, 22% effective against the A[H3N2] virus, and 50% effective against the flu B viruses. The 2019-2020 vaccine contains hemagglutinin (HA) from an A/Brisbane/02/2018(H1N1)pdm09-like virus, an A/Kansas/14/2017(H3N2)-like virus, and a B/Colorado/06/2017-like virus. The CDC also recommends receiving the vaccine before the rates of infection are high because then the body has less time to recognize the vaccine before the body is exposed to the virus. Those who are at increased risk of complications from the flu virus should take special precaution to receive their flu vaccine so that if they are exposed to the vaccine they have taken measures to prevent these types of complications. The flu can range from 2 awful weeks of body aches, stuffy nose, cough or it can lead to death which is why the vaccination and awareness of the flu should be taken very seriously.
On February 6, it was announced that the CDC has created a portable flu testing kit that cuts for on-site detection of the flu. This kit is called Mobile Influenza Analysis and it amazing because it cuts the time necessary to identify flu viruses in half. Hopefully more advances will be made in the future to help diminish the mortality rate of influenza and help those who are susceptible to complications be protected from it.
